
METASTATIC BREAST CANCER
Free webinar on Metastatic Breast Cancer, organised by The European Organisation for Research and Treatment of Cancer (EORTC) with its partner Breast International Group (BIG).
The purpose of this webinar is to raise awareness about breast cancer and the importance of academic research. The main topic will focus on the outlook for patients diagnosed with metastatic breast cancer.
The speakers are breast cancer experts and patient representatives.
When: Wednesday, 13 October 2021 from 12:00pm to 1:00pm, UK time.
Where: online.
Click here or on the picture for the full programme, and to pre-register (mandatory).
[Last updated 01 October 2021]
Free webinar on Metastatic Breast Cancer, organised by The European Organisation for Research and Treatment of Cancer (EORTC) with its partner Breast International Group (BIG).
The purpose of this webinar is to raise awareness about breast cancer and the importance of academic research. The main topic will focus on the outlook for patients diagnosed with metastatic breast cancer.
The speakers are breast cancer experts and patient representatives.
When: Wednesday, 13 October 2021 from 12:00pm to 1:00pm, UK time.
Where: online.
Click here or on the picture for the full programme, and to pre-register (mandatory).
[Last updated 01 October 2021]

ST GALLEN 2021
Free and full access to all online events from the 2021 St Gallen Breast Cancer Congress.
Click here or on the picture for access.
[Last updated 21 September 2021]
Free and full access to all online events from the 2021 St Gallen Breast Cancer Congress.
Click here or on the picture for access.
[Last updated 21 September 2021]
Did you know?
RESEARCH REVEALS ATTITUDES TO PHARMACEUTICAL INDUSTRY
New research has found positive sentiment between industry and healthcare professionals and improvement in sentiment among the public since the pandemic.
Among the findings are:
- Almost seven in ten (67 per cent) of Health Care Practitioners interviewed believe the pharmaceutical industry has supported the NHS during the pandemic.
- Over three-quarters of HCPs (76 per cent) think that the sector is doing all it can to develop solutions to end the pandemic.
- Almost two-thirds of HCPs (64 per cent) also believe the industry is continuing to invest in research for other disease areas while the pandemic continues.
- In March 2021, 60 per cent of the public said their views of the industry had improved since the pandemic. This is a significant improvement from July 2020 – the first wave of the study - when just 36 per cent said the same.
- In March 2021 more than seven in ten people (71 per cent) believe that pharmaceutical companies operating in the UK have supported the NHS during the pandemic and that they are making medicines and vaccines for COVID-19 available and affordable for everyone (68 per cent)
Click here or on the picture for details.
[Last updated 02 Aug 2021]
RESEARCH REVEALS ATTITUDES TO PHARMACEUTICAL INDUSTRY
New research has found positive sentiment between industry and healthcare professionals and improvement in sentiment among the public since the pandemic.
Among the findings are:
- Almost seven in ten (67 per cent) of Health Care Practitioners interviewed believe the pharmaceutical industry has supported the NHS during the pandemic.
- Over three-quarters of HCPs (76 per cent) think that the sector is doing all it can to develop solutions to end the pandemic.
- Almost two-thirds of HCPs (64 per cent) also believe the industry is continuing to invest in research for other disease areas while the pandemic continues.
- In March 2021, 60 per cent of the public said their views of the industry had improved since the pandemic. This is a significant improvement from July 2020 – the first wave of the study - when just 36 per cent said the same.
- In March 2021 more than seven in ten people (71 per cent) believe that pharmaceutical companies operating in the UK have supported the NHS during the pandemic and that they are making medicines and vaccines for COVID-19 available and affordable for everyone (68 per cent)
Click here or on the picture for details.
[Last updated 02 Aug 2021]

WORLD CANCER DAY
World Cancer Day every 4th February is the global uniting initiative led by the Union for International Cancer Control (UICC). By raising worldwide awareness, improving education, and catalysing personal, collective and government action, we're working together to reimagine a world where millions of preventable cancer deaths are saved and access to life-saving cancer treatment and care is equal for all - no matter who you are or where you live.
Created in 2000, World Cancer Day has grown into a positive movement for everyone, everywhere to unite under one voice to face one of our greatest challenges in history.
Each year, hundreds of activities and events take place around the world, gathering communities, organisations and individuals in schools, businesses, hospitals, marketplaces, parks, community halls, places of worship - in the streets and online - acting as a powerful reminder that we all have a role to play in reducing the global impact of cancer.
This year's World Cancer Day's theme, 'I Am and I Will', is all about you and your commitment to act. We believe that through our positive actions, together we can reach the target of reducing the number of premature deaths from cancer and noncommunicable diseases by one third by 2030.
Join us on 4 February and speak out and stand up for a cancer-free world.
Click here or on the picture for details.
[Last updated 03 February 2021]
World Cancer Day every 4th February is the global uniting initiative led by the Union for International Cancer Control (UICC). By raising worldwide awareness, improving education, and catalysing personal, collective and government action, we're working together to reimagine a world where millions of preventable cancer deaths are saved and access to life-saving cancer treatment and care is equal for all - no matter who you are or where you live.
Created in 2000, World Cancer Day has grown into a positive movement for everyone, everywhere to unite under one voice to face one of our greatest challenges in history.
Each year, hundreds of activities and events take place around the world, gathering communities, organisations and individuals in schools, businesses, hospitals, marketplaces, parks, community halls, places of worship - in the streets and online - acting as a powerful reminder that we all have a role to play in reducing the global impact of cancer.
This year's World Cancer Day's theme, 'I Am and I Will', is all about you and your commitment to act. We believe that through our positive actions, together we can reach the target of reducing the number of premature deaths from cancer and noncommunicable diseases by one third by 2030.
Join us on 4 February and speak out and stand up for a cancer-free world.
Click here or on the picture for details.
[Last updated 03 February 2021]

SIR PETER RATCLIFFE, OXFORD, AWARDED NOBLE PRIZE IN MEDICINE 2019
William G. Kaelin, MD, of Harvard University, Boston, Massachusetts, Gregg L. Semenza, MD, PhD, of Johns Hopkins University in Baltimore, Maryland, and Sir Peter J. Ratcliffe, FMedSci, of Oxford University in the United Kingdom have been awarded the 2019 Nobel Prize in Physiology or Medicine for their discoveries on how cells sense oxygen and adapt to it.
Their work can inform treatments for fighting anemia, cancer, and other diseases; the work will help better understand production of red blood cells, the generation of new blood vessels, and the fine-tuning of the immune system.
"These fundamental findings have greatly increased our understanding of how the body adapts to change," Randall Johnson, professor at the Karolinska Institute and member of the Nobel Assembly, said, according to the Associated Press (AP). "Applications of these findings are already beginning to affect the way medicine is practiced," he said.
The three scientists will share equally the cash award of $918,000
Click here or on the picture for the full press release.
[Last updated 07 October 2019]
William G. Kaelin, MD, of Harvard University, Boston, Massachusetts, Gregg L. Semenza, MD, PhD, of Johns Hopkins University in Baltimore, Maryland, and Sir Peter J. Ratcliffe, FMedSci, of Oxford University in the United Kingdom have been awarded the 2019 Nobel Prize in Physiology or Medicine for their discoveries on how cells sense oxygen and adapt to it.
Their work can inform treatments for fighting anemia, cancer, and other diseases; the work will help better understand production of red blood cells, the generation of new blood vessels, and the fine-tuning of the immune system.
"These fundamental findings have greatly increased our understanding of how the body adapts to change," Randall Johnson, professor at the Karolinska Institute and member of the Nobel Assembly, said, according to the Associated Press (AP). "Applications of these findings are already beginning to affect the way medicine is practiced," he said.
The three scientists will share equally the cash award of $918,000
Click here or on the picture for the full press release.
[Last updated 07 October 2019]
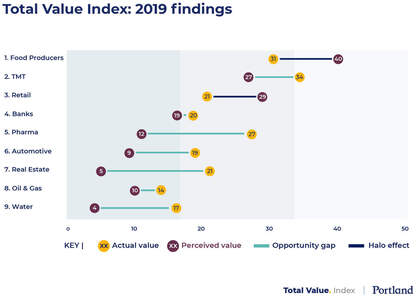
PHARMA THE LEAST SUCCESSFUL IN DEMONSTRATING ITS VALUE TO SOCIETY
The pharmaceutical industry has been ranked as one of the highest contributors to UK society, but public perception falls far below other sectors, claims a new study by Portland.
The dissonance means that the industry ranks among the least successful sectors in demonstrating its value to society, scoring consistently low on perceptions for most measures in Portland’s total value index.
Click here or on the graph to access the full report.
[Last updated 20 July 2019]
The pharmaceutical industry has been ranked as one of the highest contributors to UK society, but public perception falls far below other sectors, claims a new study by Portland.
The dissonance means that the industry ranks among the least successful sectors in demonstrating its value to society, scoring consistently low on perceptions for most measures in Portland’s total value index.
Click here or on the graph to access the full report.
[Last updated 20 July 2019]
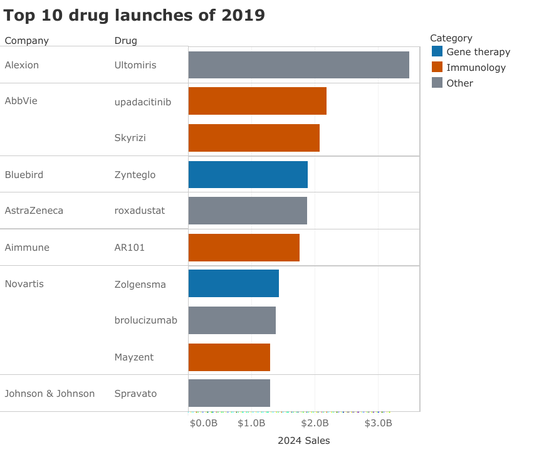
TOP-10 NEW PRODUCTS IN 2019
Ranking of this year’s top 10 drug launches by estimated 2024 sales.
Cancer is probably the most sought-after indication in the biopharma world, but it’s missing from 2019's class of top drug launches. Instead, treatments in immunology and rare genetic diseases have grabbed the limelight.
Click here or on the picture for details of this new analysis.
[Last updated 02 July 2019]
Ranking of this year’s top 10 drug launches by estimated 2024 sales.
Cancer is probably the most sought-after indication in the biopharma world, but it’s missing from 2019's class of top drug launches. Instead, treatments in immunology and rare genetic diseases have grabbed the limelight.
Click here or on the picture for details of this new analysis.
[Last updated 02 July 2019]

NEW GUIDANCE ON WORKING WITH PATIENTS
The ABPI has published a new guide for pharmaceutical companies working with patients.
The guide Working with patients and patient organisations - a sourcebook for industry provides companies with new guidance on working successfully, collaboratively and ethically with patients and patient groups and in line with the ABPI Code of Practice.
It’s also a response to patient organisations looking for greater clarity on their relationships with companies and confirms the ABPI view that working with patients and patient organisations can bring significant public health benefits.
Click here or on the picture to get a copy of the new guide.
[Last updated 21 June 2019]
The ABPI has published a new guide for pharmaceutical companies working with patients.
The guide Working with patients and patient organisations - a sourcebook for industry provides companies with new guidance on working successfully, collaboratively and ethically with patients and patient groups and in line with the ABPI Code of Practice.
It’s also a response to patient organisations looking for greater clarity on their relationships with companies and confirms the ABPI view that working with patients and patient organisations can bring significant public health benefits.
Click here or on the picture to get a copy of the new guide.
[Last updated 21 June 2019]
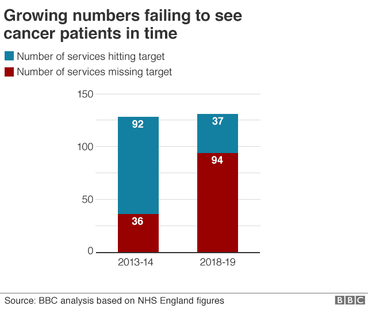
THE WORST PLACES FOR CANCER CARE WAIT
Nearly three-quarters of services are failing to treat cancer patients quickly enough.
Hospitals are meant to start treatment within 62 days of an urgent referral by a GP in 85% of cases.
But 94 out of 131 cancer services in England failed to do that during 2018-19 - compared with 36 just five years ago.
Overall, across England, more than 32,000 patients waited longer than 62 days for treatment to start.
Click here or on the picture for more details.
[Last updated 13 June 2019]
Nearly three-quarters of services are failing to treat cancer patients quickly enough.
Hospitals are meant to start treatment within 62 days of an urgent referral by a GP in 85% of cases.
But 94 out of 131 cancer services in England failed to do that during 2018-19 - compared with 36 just five years ago.
Overall, across England, more than 32,000 patients waited longer than 62 days for treatment to start.
Click here or on the picture for more details.
[Last updated 13 June 2019]

SEIZING THE OPPORTUNITIES FOR BREAST CANCER PATIENTS
A group of members of the European Parliament is promoting a manifesto to make breast cancer a top priority in EU.
The key recommendations of the “European Elections Manifesto 2019: Seizing the Opportunities for Breast Cancer Patients”, are:
1. Ensuring breast cancer is screened, diagnosed and treated at an early stage and that all breast cancer patients have access to treatment in a breast unit by a specialized multidisciplinary team.
2. Ensuring return to work programmes for breast cancer patients and survivors.
3. Maintaining a favourable environment for the collection of breast cancer data and the development of innovative health technologies.
Click here or on the picture to access the full manifesto.
[Last updated 10 June 2019]
A group of members of the European Parliament is promoting a manifesto to make breast cancer a top priority in EU.
The key recommendations of the “European Elections Manifesto 2019: Seizing the Opportunities for Breast Cancer Patients”, are:
1. Ensuring breast cancer is screened, diagnosed and treated at an early stage and that all breast cancer patients have access to treatment in a breast unit by a specialized multidisciplinary team.
2. Ensuring return to work programmes for breast cancer patients and survivors.
3. Maintaining a favourable environment for the collection of breast cancer data and the development of innovative health technologies.
Click here or on the picture to access the full manifesto.
[Last updated 10 June 2019]
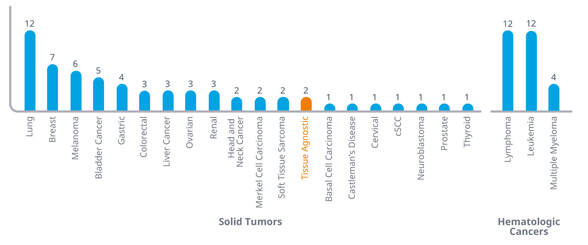
GLOBAL ONCOLOGY TRENDS 2019
A record number of new oncology drugs has been approved in recent years, bringing new treatment options to patients. However, despite robust levels of pipeline activity, oncology remains a challenging area for research and development. A new report examines the productivity and output of the oncology pipeline, new treatment mechanisms, and which patients will likely benefit from new therapies.
Click here or on the picture to claim your copy of the report.
[Last updated 08 June 2019]
A record number of new oncology drugs has been approved in recent years, bringing new treatment options to patients. However, despite robust levels of pipeline activity, oncology remains a challenging area for research and development. A new report examines the productivity and output of the oncology pipeline, new treatment mechanisms, and which patients will likely benefit from new therapies.
Click here or on the picture to claim your copy of the report.
[Last updated 08 June 2019]
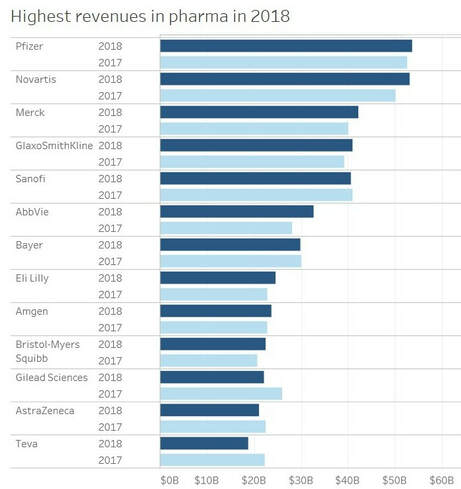
TOP-15 PHARMACEUTICAL COMPANIES 2018
The pharma industry is always in flux, but the big names always turn up near the top of yearly sales rankings. This year is no different, with Johnson & Johnson, Roche, Pfizer, Novartis and Merck & Co. taking the top 5 spots in Big Pharma companies by 2018 sales.
In fact, the top 15 names are the same against prior year rankings, but the order has shifted. Last year’s No. 5, Sanofi, slipped to No. 7 as its sales sank almost 2% year over year. Gilead Sciences, famously under hepatitis C pricing pressure for years, sank even farther in 2018, slipping three positions to No. 13.
On the flip side, Bristol-Myers Squibb climbed from No. 15 to No. 12. And if the company scores its massive Celgene buyout, it’ll be even higher next year. Adding Celgene’s $15 billion in 2018 revenue would have given BMS nearly $38 billion in annual sales, ranking it among the top 10 pharma companies by annual sales.
Click here or on the picture to read more about this story.
[Last updated 08 April 2019]
The pharma industry is always in flux, but the big names always turn up near the top of yearly sales rankings. This year is no different, with Johnson & Johnson, Roche, Pfizer, Novartis and Merck & Co. taking the top 5 spots in Big Pharma companies by 2018 sales.
In fact, the top 15 names are the same against prior year rankings, but the order has shifted. Last year’s No. 5, Sanofi, slipped to No. 7 as its sales sank almost 2% year over year. Gilead Sciences, famously under hepatitis C pricing pressure for years, sank even farther in 2018, slipping three positions to No. 13.
On the flip side, Bristol-Myers Squibb climbed from No. 15 to No. 12. And if the company scores its massive Celgene buyout, it’ll be even higher next year. Adding Celgene’s $15 billion in 2018 revenue would have given BMS nearly $38 billion in annual sales, ranking it among the top 10 pharma companies by annual sales.
Click here or on the picture to read more about this story.
[Last updated 08 April 2019]
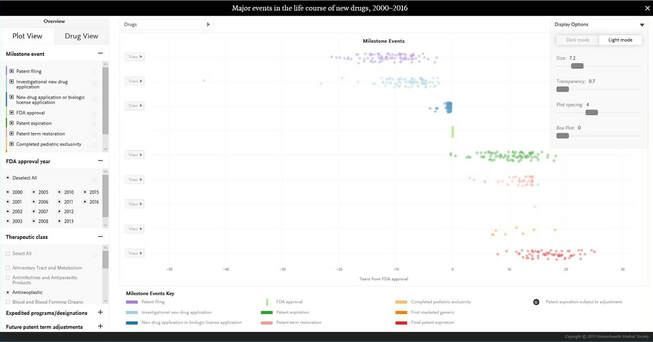
MAJOR EVENTS IN THE LIFE COURSE OF NEW DRUGS, 2000–2016
An interactive graphic allowing viewers to explore data — gathered by Reed F. Beall, Thomas J. Hwang, and Aaron S. Kesselheim — on the time required for investigational drugs to reach important U.S. milestones, such as new drug applications, FDA approval, expiration of market exclusivity, and market entry of a generic version.
Click here or on the picture to access the model.
[Last updated 28 March 2019]
An interactive graphic allowing viewers to explore data — gathered by Reed F. Beall, Thomas J. Hwang, and Aaron S. Kesselheim — on the time required for investigational drugs to reach important U.S. milestones, such as new drug applications, FDA approval, expiration of market exclusivity, and market entry of a generic version.
Click here or on the picture to access the model.
[Last updated 28 March 2019]

New version: The 2019 ABPI/PMCPA Code of Practice for the Pharmaceutical Industry issued.
The Code sets standards for the promotion of medicines to health professionals and other relevant decision makers in the UK. It includes requirements for the provision of information to patients and the public and relationships with patient groups. The Code also applies to a number of areas that are non-promotional.
The detailed provisions in the Code aim to ensure that pharmaceutical companies operate in a responsible, ethical and professional manner. The Code covers:
- journal, direct mail and digital advertising
- the activities of representatives, including any materials used by them
the supply of samples
- the provisions of inducements to prescribe, supply, administer, buy or sell medicines, by the gift, offer or promise of any benefit or bonus whether in money or in kind
- the provision of hospitality
- promotional meetings
- the sponsorship of scientific and other meetings including payment of travel and accommodation expenses
- all other sales promotion including exhibitions and digital communications
the provision of information to the public
- relationships with patient organisations.
Even if the 2019 version takes effect from 1st January 2019, PMCPA has issued a grace period until 30 April 2019 where no material or activity will be regarded as breaching the Code if failing to comply with any revised elements; however, the requirements of the current version (2016) must then be adhered to.
Click here or on the picture for details.
[Last updated 09 December 2018]
The Code sets standards for the promotion of medicines to health professionals and other relevant decision makers in the UK. It includes requirements for the provision of information to patients and the public and relationships with patient groups. The Code also applies to a number of areas that are non-promotional.
The detailed provisions in the Code aim to ensure that pharmaceutical companies operate in a responsible, ethical and professional manner. The Code covers:
- journal, direct mail and digital advertising
- the activities of representatives, including any materials used by them
the supply of samples
- the provisions of inducements to prescribe, supply, administer, buy or sell medicines, by the gift, offer or promise of any benefit or bonus whether in money or in kind
- the provision of hospitality
- promotional meetings
- the sponsorship of scientific and other meetings including payment of travel and accommodation expenses
- all other sales promotion including exhibitions and digital communications
the provision of information to the public
- relationships with patient organisations.
Even if the 2019 version takes effect from 1st January 2019, PMCPA has issued a grace period until 30 April 2019 where no material or activity will be regarded as breaching the Code if failing to comply with any revised elements; however, the requirements of the current version (2016) must then be adhered to.
Click here or on the picture for details.
[Last updated 09 December 2018]

October is Breast Cancer Awareness Month
A worldwide annual campaign involving thousands of organisations, Breast Cancer Awareness Month highlights the importance of breast awareness, education and research.
A number of activities are taking place at the Annual European Society for Medical Oncology in Munich, Germany – click here for details.
Click here or on the picture to learn more about Breast Cancer Awareness Month.
[Last updated 18 October 2018]
A worldwide annual campaign involving thousands of organisations, Breast Cancer Awareness Month highlights the importance of breast awareness, education and research.
A number of activities are taking place at the Annual European Society for Medical Oncology in Munich, Germany – click here for details.
Click here or on the picture to learn more about Breast Cancer Awareness Month.
[Last updated 18 October 2018]
Eli Lilly’s CDK 4 and 6 inhibitor abemaciclib (Verzenios) has been approved in Europe for the treatment of certain metastatic breast cancers.
The decision gives doctors the green light to prescribe the drug for women with hormone receptor-positive (HR+), epidermal growth factor receptor 2 negative (HER2–) locally advanced or metastatic breast cancer, in combination with an aromatase inhibitor (AI) or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy.
The applications were based on the Phase III MONARCH 2 and 3 trials.
In MONARCH 2, adding abemaciclib to AstraZeneca’s Faslodex (fulvestrant) significantly improved progression-free survival compared to Faslodex plus placebo, with figures of 16.4 months versus 9.3 months, respectively.
MONARCH 3 evaluated abemaciclib in combination with an AI as initial endocrine-based therapy in postmenopausal women with HR+, HER2– advanced breast cancer who had no prior systemic treatment for advanced disease.
The data show that combining the drug with an AI demonstrated a greater than 28-month median PFS in patients who received initial endocrine-based therapy for metastatic disease (28.2 months vs 14.8 months for placebo/AI).
Click here or on the picture for details.
[Last updated 07 October 2018]
The decision gives doctors the green light to prescribe the drug for women with hormone receptor-positive (HR+), epidermal growth factor receptor 2 negative (HER2–) locally advanced or metastatic breast cancer, in combination with an aromatase inhibitor (AI) or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy.
The applications were based on the Phase III MONARCH 2 and 3 trials.
In MONARCH 2, adding abemaciclib to AstraZeneca’s Faslodex (fulvestrant) significantly improved progression-free survival compared to Faslodex plus placebo, with figures of 16.4 months versus 9.3 months, respectively.
MONARCH 3 evaluated abemaciclib in combination with an AI as initial endocrine-based therapy in postmenopausal women with HR+, HER2– advanced breast cancer who had no prior systemic treatment for advanced disease.
The data show that combining the drug with an AI demonstrated a greater than 28-month median PFS in patients who received initial endocrine-based therapy for metastatic disease (28.2 months vs 14.8 months for placebo/AI).
Click here or on the picture for details.
[Last updated 07 October 2018]
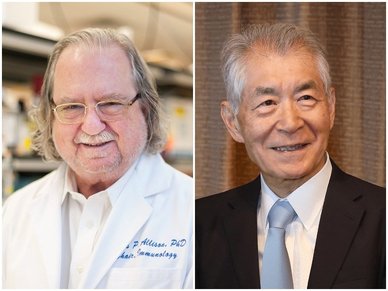
Nobel Prize 2018
US researcher James Allison and Japanese researcher Tasuku Honjo have won the 2018 Nobel Prize for Physiology or Medicine for their ground-breaking work on manipulating the immune system to combat cancer.
“By stimulating the inherent ability of our immune system to attack tumour cells this year’s Nobel Laureates have established an entirely new principle for cancer therapy,” said the Nobel Assembly at Sweden’s Karolinska Institute.
Allison studied a known protein that functions as a brake on the immune system, and realised the potential of releasing the brake and thereby unleashing our immune cells to attack tumours. He then developed this concept into a brand-new approach for treating patients.
Tasuku Honjo discovered a protein on immune cells and, after exploring its function, eventually revealed that it also operates as a brake, but with a different mechanism of action. Therapies based on his discovery proved to be strikingly effective in the fight against cancer.
D2MM congratulates them both – and the entire scientific world – with these ground-breaking discoveries which have led to so many patient benefits.
Click here or on the picture to see the The Noble Prize Committees announcement.
[Last updated 07 October 2018]
US researcher James Allison and Japanese researcher Tasuku Honjo have won the 2018 Nobel Prize for Physiology or Medicine for their ground-breaking work on manipulating the immune system to combat cancer.
“By stimulating the inherent ability of our immune system to attack tumour cells this year’s Nobel Laureates have established an entirely new principle for cancer therapy,” said the Nobel Assembly at Sweden’s Karolinska Institute.
Allison studied a known protein that functions as a brake on the immune system, and realised the potential of releasing the brake and thereby unleashing our immune cells to attack tumours. He then developed this concept into a brand-new approach for treating patients.
Tasuku Honjo discovered a protein on immune cells and, after exploring its function, eventually revealed that it also operates as a brake, but with a different mechanism of action. Therapies based on his discovery proved to be strikingly effective in the fight against cancer.
D2MM congratulates them both – and the entire scientific world – with these ground-breaking discoveries which have led to so many patient benefits.
Click here or on the picture to see the The Noble Prize Committees announcement.
[Last updated 07 October 2018]
LARGEST PHARMACEUTICAL COMPANIES
The top 10 drugmakers are expected to hold 35% of the total biopharma market share by 2024, or more than $420 billion in sales. This is according to a new report by EvaluatePharma.
The top 10 drugmakers of 2024:
1. Novartis
2. Pfizer
3. Roche
4. Johnson & Johnson
5. Sanofi
6. GlaxoSmithKline
7. Merck
8. AbbVie
9. AstraZeneca
10. Bristol-Myers Squibb
Click here – or on each company name – for details.
[Last updated 30 September 2018]
The top 10 drugmakers are expected to hold 35% of the total biopharma market share by 2024, or more than $420 billion in sales. This is according to a new report by EvaluatePharma.
The top 10 drugmakers of 2024:
1. Novartis
2. Pfizer
3. Roche
4. Johnson & Johnson
5. Sanofi
6. GlaxoSmithKline
7. Merck
8. AbbVie
9. AstraZeneca
10. Bristol-Myers Squibb
Click here – or on each company name – for details.
[Last updated 30 September 2018]

FOUR PHARMA COMPANIES NAMED FOR BREACHING ABPI CODE OF PRACTICE
Martindale Pharma, Pierre Fabre, Janssen and Pharmasure have all been named in advertisements for breaching the Association of the British Pharmaceutical Industry’s Code of Practice.
According to the ABPI’s Prescription Medicines Code of Practice Authority (PMCPA), all have brought discredit upon and reduced confidence in the pharmaceutical industry.
Pierre Fabre was reprimanded for briefing representatives about Toviaz (fesoterodine) “using an uncertified presentation and failing to provide complete information,” the PMCPA said, and thus breached four Code clauses, including: Clause 14.1, failing to certify promotional material; and Clause 15.9, producing representatives’ briefing material that did not comply with the relevant requirements of the Code.
The firm was also found to have breached seven clauses of the Code by failing to certify representatives’ briefing materials and one presentation aimed at health professionals which promoted an unlicensed medicine, including briefing materials related to Toviaz that were misleading, and “not capable of substantiation”.
For advertising Espranor oral lyophilisate (buprenorphine) to the public and for making claims in material aimed at patients and health professionals that were “prejudicial to patient safety”, Martindale was found to have breached eight clauses of the Code.
These include Clause 9.1, failing to maintain high standards, Clause 7.2, providing misleading information, and Clause 26.1, advertising a prescription only medicine to the public.
Janssen broke rules by promoting a medicine prior to it having received authorisation, breaking three clauses, while for providing a group of health professionals with a hamper of chocolates, Pharmasure also breached three requirements of the Code, including Clause 18.1, providing a gift in connection with the promotion of medicines.
Click here or on the picture for details.
[Last updated 24 July 2018]
Martindale Pharma, Pierre Fabre, Janssen and Pharmasure have all been named in advertisements for breaching the Association of the British Pharmaceutical Industry’s Code of Practice.
According to the ABPI’s Prescription Medicines Code of Practice Authority (PMCPA), all have brought discredit upon and reduced confidence in the pharmaceutical industry.
Pierre Fabre was reprimanded for briefing representatives about Toviaz (fesoterodine) “using an uncertified presentation and failing to provide complete information,” the PMCPA said, and thus breached four Code clauses, including: Clause 14.1, failing to certify promotional material; and Clause 15.9, producing representatives’ briefing material that did not comply with the relevant requirements of the Code.
The firm was also found to have breached seven clauses of the Code by failing to certify representatives’ briefing materials and one presentation aimed at health professionals which promoted an unlicensed medicine, including briefing materials related to Toviaz that were misleading, and “not capable of substantiation”.
For advertising Espranor oral lyophilisate (buprenorphine) to the public and for making claims in material aimed at patients and health professionals that were “prejudicial to patient safety”, Martindale was found to have breached eight clauses of the Code.
These include Clause 9.1, failing to maintain high standards, Clause 7.2, providing misleading information, and Clause 26.1, advertising a prescription only medicine to the public.
Janssen broke rules by promoting a medicine prior to it having received authorisation, breaking three clauses, while for providing a group of health professionals with a hamper of chocolates, Pharmasure also breached three requirements of the Code, including Clause 18.1, providing a gift in connection with the promotion of medicines.
Click here or on the picture for details.
[Last updated 24 July 2018]

CODE OF CONDUCT BREACHES
Breaches by pharmaceuticals companies of the prescription medicines code have risen sharply.
The number of complaints received by the Prescription Medicines Code of Practice Authority rose by 41 per cent to 76 last year, its annual report shows. This led to 100 cases, a 52 per cent increase for the authority, which found 57 breaches of the code that governs standards for the promotion of medicines, a 63 per cent rise on 2015.
The companies involved included GlaxoSmithKline and AstraZeneca, Britain’s biggest pharmaceuticals businesses, Novartis, Pfizer and Merck.
Click here to access the latest Annual Report from the Prescription Medicines Code of Practice Authority (PMCPA).
[Last updated 30 October 2017]
Breaches by pharmaceuticals companies of the prescription medicines code have risen sharply.
The number of complaints received by the Prescription Medicines Code of Practice Authority rose by 41 per cent to 76 last year, its annual report shows. This led to 100 cases, a 52 per cent increase for the authority, which found 57 breaches of the code that governs standards for the promotion of medicines, a 63 per cent rise on 2015.
The companies involved included GlaxoSmithKline and AstraZeneca, Britain’s biggest pharmaceuticals businesses, Novartis, Pfizer and Merck.
Click here to access the latest Annual Report from the Prescription Medicines Code of Practice Authority (PMCPA).
[Last updated 30 October 2017]
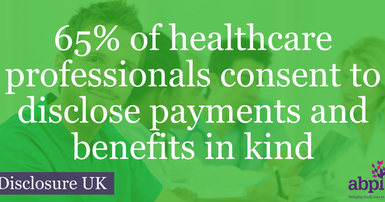
HEALTHCARE PROFESSIONALS WORKING IN PARTNERSHIP WITH THE PHARMACEUTICAL INDUSTRY
In 2016 the pharmaceutical industry spent a total of £454.5m working in partnership with leading UK health experts and organisations to improve patient care – a 25% increase from 2015 (£363m).
This rise is driven by an increase in payments relating to research and development shown in aggregate, which has increased 33% from 2015 (£254m) to £338.1m in 2016. 74% of payments to HCPs and HCOs in 2016 relate to research and development.
The remaining £116.5m (£109m in 2015) was for payments and benefits in kind not related to research and development.
Click here for more information.
[Last updated 01 July 2017]
In 2016 the pharmaceutical industry spent a total of £454.5m working in partnership with leading UK health experts and organisations to improve patient care – a 25% increase from 2015 (£363m).
This rise is driven by an increase in payments relating to research and development shown in aggregate, which has increased 33% from 2015 (£254m) to £338.1m in 2016. 74% of payments to HCPs and HCOs in 2016 relate to research and development.
The remaining £116.5m (£109m in 2015) was for payments and benefits in kind not related to research and development.
Click here for more information.
[Last updated 01 July 2017]
NICE to fast-track cheaper medicines; however, drugs that will cost more than £20m in any one of their first three years of use will trigger commercial discussions between the company and NHS England to mitigate the impact on the rest of the NHS.
ABPI (Association of British Pharmaceutical Indistry) objects to these changes.
Click here or on the picture for more information from NICE, and here for ABPI's reaction.
[Last updated 15 March 2017]
ABPI (Association of British Pharmaceutical Indistry) objects to these changes.
Click here or on the picture for more information from NICE, and here for ABPI's reaction.
[Last updated 15 March 2017]

Four named pharmaceutical companies (Vifor Pharma, Celgene, Takeda and Pierre Fabre) have all been found in breach of the most serious clause in the ABPI Code of Conduct, Clause 2, which means that they are bringing discredit upon, and reducing confidence in, the pharmaceutical industry.
Click here for more information about the PMCPA’s (Prescription Medicines Code of Practice Authority) very serious rulings.
Contact us if want to avoid your company ending up in the same situation.
[Last updated 14 March 2017]
Click here for more information about the PMCPA’s (Prescription Medicines Code of Practice Authority) very serious rulings.
Contact us if want to avoid your company ending up in the same situation.
[Last updated 14 March 2017]

Please see the Accelerated Access Review (AAR) which was published today. It was commissioned by the government in November 2014. The final report makes recommendations to make it easier for NHS patients to access innovative medicines, medical technologies, diagnostics and digital products, improving efficiency and patient outcomes.
The AAR has been led by an independent chair, Sir Hugh Taylor, supported by Sir John Bell as Chair of the External Advisory Group. There has been extensive engagement with industry, the NHS, patients, academia and clinicians during its development.
The AAR has been led by an independent chair, Sir Hugh Taylor, supported by Sir John Bell as Chair of the External Advisory Group. There has been extensive engagement with industry, the NHS, patients, academia and clinicians during its development.

Code of Practice for the Pharmaceutical Industry
In 2016 pharmaceutical companies will disclose details of certain transfers of value to named health professionals, other relevant decision makers and organisations made during 2015. See Clause 24 in the ABPI Code of Practice for the Pharmaceutical Industry; alternatively, Click here for a tutorial.
In 2016 pharmaceutical companies will disclose details of certain transfers of value to named health professionals, other relevant decision makers and organisations made during 2015. See Clause 24 in the ABPI Code of Practice for the Pharmaceutical Industry; alternatively, Click here for a tutorial.

